Validating Methenamine Synthesis using Raman Spectroscopy
One of the main reasons I started the OpenRAMAN project was to enable amateur chemists and students to validate synthesis they used in their home/pet projects. Without metrology instruments, it is impossible to perform science correctly and chemistry is no exception to that. I already discussed the “blind faith” approach of chemistry in my former post about bisulfite synthesis.
Methenamine synthesis is probably one of the most well-known reaction in the amateur chemistry world because it requires only household reagents and basic apparatus. The synthesis is relatively straightforward and has the reputation of yielding a relatively pure product. The compound has fairly large usage spectrum in the industry, ranging from polymers preparation to medical usage for urinary tract infections. In the amateur world, methenamine is mostly known for its energetic material applications as it is the primary reagent for the preparation of RDX. For further readings on methenamine, I can recommend the Wikipedia and ScienceMadness pages.
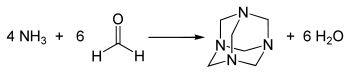
The synthesis of methenamine is briefly described on the Wikipedia page and is copied here in Figure 1. 4 moles of ammoniac will react with 6 moles of formaldehyde to yield one mole of methenamine. In amateur labs, the reaction is performed in wet condition and ammonia is therefore substituted to ammoniac gas. Note how the bonds evolve between the reagents and the products.
The synthesis I designed was directly derived from the reaction itself. It is not original as most people work using a similar protocol.
25 ml of 12% ammonia were slowly added to 10 ml of 29% formaldehyde using a magnetic stirrer. The solution is then slowly boiled off to remove the excess water in which the methenamine is soluble. Finally, a recrystallisation step is performed from hot 85% ethanol.
Some notes should be made about this synthesis. First, and despite both ammonia and formaldehyde can be obtained as household reagents, they still are pretty toxic/harmful. Good ventilation and safety equipment is mandatory to perform this synthesis. I recommend forced air ventilation and eye protection at any time. Be also very cautious during the last part of the evaporation to avoid projections of the synthesized products or its decomposition which can severely hurt you. A slight excess of ammonia is also required because you don’t want to boil off formaldehyde fumes which are both toxic and carcinogenic (reason for which I used 25 ml of ammonia instead of the ~21 ml required). Generally speaking, do not perform this synthesis if you don’t fully understand what you are doing, and the risks associated to the chemicals you are using. The here-above synthesis is only a summarized version and does not substitute to a suitable academic training in chemistry.
After performing the synthesis, I compared the crystals to a pure sample of methenamine obtained from a chemical supplier (99.9% pure). The two spectra, experimental and reference, are shown in Figure 2 and were taken using the OpenRAMAN Starter Edition. I still haven’t replaced the laser of the Performance Edition since I fried it and I therefore have to perform all the analysis using the Starter Edition which has a multimode laser leading to poorer resolution and doubling of the peaks. Nonetheless, the spectra shown here are a good example of what can be obtained using the entry level version of the spectrometer using the small 5 mW laser at 11 sec exposure (no baseline removal). It is therefore perfectly applicable by the amateur chemist.
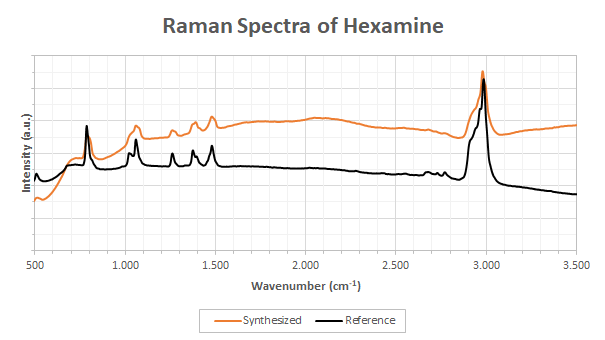
The results of Figure 2 tends to show that we are dealing with the same compound (synthesized vs. reference) but the synthesized methenamine has a broader fluorescence spectrum. This fluorescence background is actually the consequence of the impurities in the ethanol I used which is something I did not anticipated when performing the recrystallization.
Let us look at the spectra in more details using a first order Savitzky-Gollay filtering. I already discussed the advantages of first order filtering in my post about esterification. The results are shown in Figure 3.
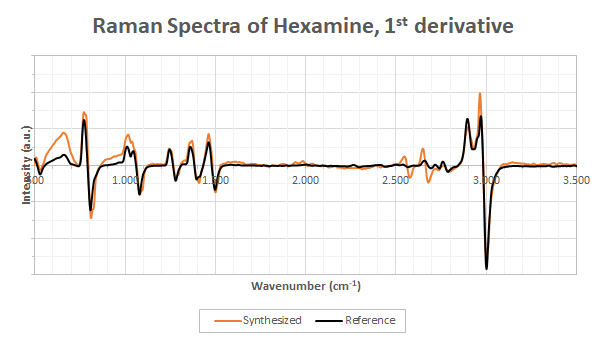
The peaks are clearly the same which confirms that the product synthesized is indeed methenamine. However, something interesting occurs in the region 2,500-3000 cm-1. First, there is clearly some extra peaks near 2,600 cm-1 but the peak at 3,000 cm-1 seems to be a bit stronger in the synthesized compound too. This means, without any doubts, that the synthesized product I’m analyzing is not as pure as the reference version. This may come from the impurities in the recrystallisation process although the crystals I analyzed were still a bit damp and I cannot exclude that not all the solvent evaporated when I performed the analysis.
Concerning the peak positions, we can exclude any contamination of C#N (triple bonds) as they would have appeared in the 2,000-2,300 cm-1 region which is empty in our spectra. The peaks near 1,000-1,500 cm-1 are most probably the C-N and CH2 bonds of methenamine. Although I do not have the N(R)3 peaks in my tables, NH2R occurs near 1,050 cm-1 and NHR2 near 1,150 cm-1. C-N are also expected to provide a signal near 900 cm-1. The peaks near 1,500 cm-1 are most probably due to CH2 bonds. As always, be aware that I have very little experience in peak identifications so do not take my words here for granted.
So, what shall we conclude from all of this? Mostly, a methodology. In the bisulfite synthesis post, I showed how we could draw conclusions based on an analysis of the spectra of the reagents and the product. Here, I showed how a 1st derivative analysis of a product compared to some reference material could show minute discrepancies and highlight contaminations in a protocol. To be complete, we should have iterated on our synthesis and show that our modifications resulted in an increase of quality.
This approach is what draws the line between cook and chemistry. Enabling chemists, amateur or students, to think in a critical way about their work is at the core of the OpenRAMAN project.
I would like to give a big thanks to James, Lilith, Cam, Samuel, Themulticaster, Sivaraman, Vaclav, Arif, Jesse and Jon who have supported this post through Patreon. I remind you to take the occasion to invite you to donate through Patreon, even as little as $1. I cannot stress it more, you can really help me to post more content and make more experiments! The device presented in this post was paid 100% through the money collected on Patreon!
0 Comments